SPR1NT was an open-label, single-arm clinical trial of presymptomatic patients with SMA1
All patients enrolled in the study were less than 6 weeks of age and did not display any symptoms of SMA at the time of infusion. The SPR1NT trial was divided into 2 cohorts based on SMN2 copy number—patients with 2 copies of SMN2 and those with 3 copies of SMN2.1
Study results for patients with 2 copies of SMN2 (n=14)1
Presymptomatic patients with 2 copies of SMN2 achieved age-appropriate motor milestones1
Motor milestone achievements at or before the 18-month-of-age study visit1
Primary endpoint
100% (14/14) achieved sitting without support for ≥30 seconds (Bayley)1,a
11 of those 14 patients achieved this milestone within an age-appropriate time period1,5
aBayley-III, gross motor subtest item 26. WHO MGRS established windows of achievement (1%–99%): 3.8–9.2 months for sitting without support.2,5
bIndependent standing ≥10 seconds assessed by WHO MGRS. WHO MGRS established window of achievement (1%–99%): 6.9–16.9 months for standing alone.2,5
cIndependent walking assessed by WHO MGRS. WHO MGRS established windows of achievement (1%–99%): 8.2–17.6 months for walking alone.2,5
ZOLGENSMA enabled continued independence from respiratory and nutritional support in presymptomatic patients with 2 copies of SMN21
Study results for patients with 3 copies of SMN2 (n=15)3
Presymptomatic patients with 3 copies of SMN2 achieved age appropriate motor milestones3
Motor milestone achievements at or before the 24-month-of-age study visit3
aBayley-III, gross motor subtest item 40. WHO MGRS established windows of achievement (1%–99%): 6.9–16.9 months for standing alone.3,5
bBayley-III, gross motor subtest item 43. WHO MGRS established windows of achievement (1%–99%): 8.2–17.6 months for walking alone.3,5
ZOLGENSMA enabled independence from respiratory and nutritional support in presymptomatic patients with 3 copies of SMN23
aAll patients were required to be able to swallow thing liquids and be free from ventilatory support at baseline. Ventilatory support included noninvasive ventilatory support, invasive ventilatory support, cough assist, or Bilevel positive airway pressure (BiPAP).
LT-002 is a long-term follow-up study that includes patients from SPR1NT
LT-002 is a 15-year, ongoing, open-label, long-term follow-up of patients who completed a ZOLGENSMA clinical trial. It includes patients with SMA who were either symptomatic or presymptomatic at the time of infusion. Twenty-five patients from the SPR1NT trial have enrolled, including 12 from the 2-copy cohort and 13 from the 3-copy cohort.6,7
The following data are from patients from SPR1NT, who were presymptomatic for SMA at the time of infusion.
In long-term follow-up, ZOLGENSMA continued to provide durable efficacy over 3 years post dose
All patients were alive, and none required permanent ventilation in the ongoing trial (as of May 2022). The mean time since treatment was 3.5 (range 2.9–4.1) years and 3.2 (range 2.8–3.7) years for patients with 2 and 3 copies of SMN2, respectively.6
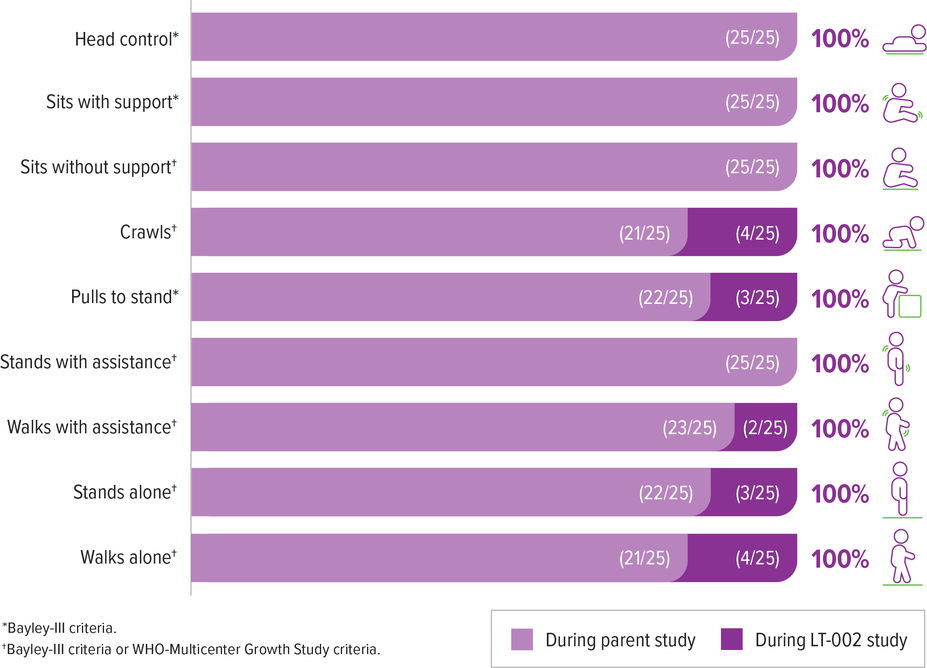
Patients achieved and maintained all motor milestones assessed in the presymptomatic SPR1NT study6,a
All 4 patients who did not achieve walking alone in the SPR1NT study achieved this milestone during LT-002 (as of May 2022).6
2 of these 4 patients had received other add-on therapy during LT-002 (1 of these 2 patients achieved walking alone before receiving add-on therapy)6,b
aIn long-term follow-up some milestone achievements occurred after add-on therapy: crawls (2/4), pulls to stand (2/3), walks with assistance (1/2), stands alone (1/3), walks alone (1/4).
bAdd-on was defined by treatment with any other disease-modifying therapy.