Efficacy: STR1VE trial
STR1VE: An open-label, single-arm clinical trial that demonstrated the efficacy of ZOLGENSMA1
STR1VE was a Phase 3 trial of symptomatic patients with SMA Type 1 (N=22)1,a
Patients were symptomatic and less than 6 months of age at the time of infusion and studied until 18 months of age.
aOne patient was initially classified as presymptomatic and removed from the intent-to-treat (ITT) data set included in the Prescribing Information. The patient has been confirmed to be symptomatic at baseline and included in the final ITT analysis.2
EXPAND ENDPOINTS AND ENROLLMENT CRITERIA
Endpoints1,2
Primary endpoints:
-
Event-free survival at 14-months-of-age study visita
-
Functional, independent sitting for ≥30 seconds at 18-months-of-age study visit
Secondary endpoints:
- Ability to thrive at 18 months of age, a composite endpoint: maintains weight (≥3rd percentile for age and gender, based on World Health Organization Child Growth Standards), does not receive nutrition through mechanical support, and has the ability to tolerate thin liquids as demonstrated through a formal swallowing test
- Independence from ventilatory support at 18 months of age (based solely on Trilogy BiPAP data)
Exploratory endpoints:
-
Change from baseline in Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND)
-
Other motor milestone achievements
Key inclusion and exclusion criteria1
Key inclusion criteria:
- <6 months of age at the time of infusion
- Bi-allelic SMN1 mutations and one or two copies of SMN2 (inclusive of SMN2 gene modifier [c.859G>C])b
Key exclusion criteria:
- Anti-AAV9 antibody titers >1:50
- Tracheostomy or current use of noninvasive ventilatory support averaging ≥6 hours/day over 7 days prior to screening
- Active viral infection
- Treatment with an investigational or commercial product given for the treatment of SMA
aEvent is defined as death or the need for permanent ventilatory support defined by tracheostomy or by the requirement of ≥16 hours of respiratory assistance per day continuously for ≥14 days in the absence of an acute reversible illness, excluding perioperative ventilation.1
bWhile the protocol allowed inclusion of patients with the SMN2 gene modifier mutation (c.859G>C) the ITT population excludes such patients. None of the 22 patients enrolled in STR1VE had this genetic modifier.2
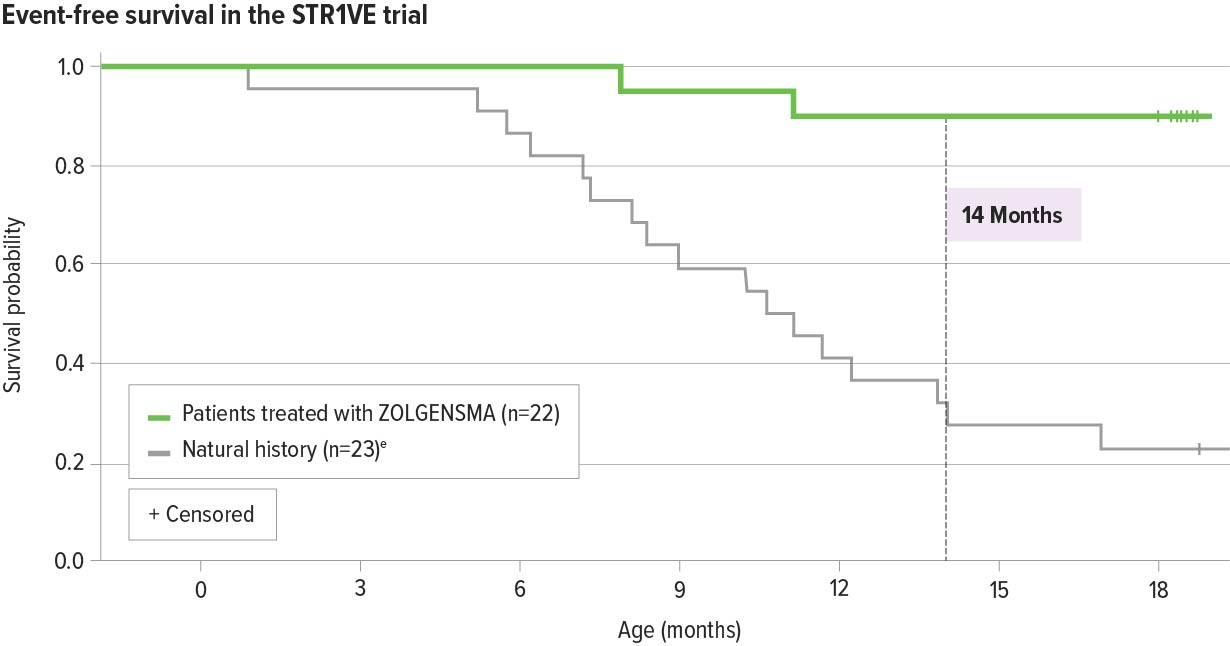
ZOLGENSMA significantly increased event-free survival in patients with SMA Type 1 compared with natural history2
91% (20/22) of patients were alive and free of permanent ventilation at the 14-months-of-age study visit, a primary endpoint2,a-c
- Results were maintained through 18 months of age2,d
aEvent is defined as death or the need for permanent ventilatory support consisting of ≥16 hours of respiratory assistance per day continuously for ≥14 days.2
bOne patient was initially classified as presymptomatic and removed from the intent-to-treat (ITT) data set included in the Prescribing Information. The patient has been confirmed to be symptomatic at the time of gene therapy and included in the final ITT analysis.2
cOne patient died at 7.8 months from respiratory failure, which was deemed unrelated to treatment. One patient withdrew consent at 11.9 months of age; this patient was determined by the investigator to have required permanent ventilation at the time of discontinuation.2
dOne patient discontinued participation at the age of 18.0 months, before the month 18 end-of-study-visit, due to an adverse event of respiratory distress (considered unrelated to study drug). The patient was alive without permanent ventilation at the time of withdrawal.2
eNatural history: The Pediatric Neuromuscular Clinical Research (PNCR) Network study population, with bi-allelic deletion of SMN1 gene, 2 copies of SMN2, and onset of SMA symptoms at age ≤6 months, was used as a matched control cohort for START and STR1VE studies.3
aEvent is defined as death or the need for permanent ventilatory support consisting of ≥16 hours of respiratory assistance per day continuously for ≥14 days.2
bOne patient was initially classified as presymptomatic and removed from the intent-to-treat (ITT) data set included in the Prescribing Information. The patient has been confirmed to be symptomatic at the time of gene therapy and included in the final ITT analysis.2
cOne patient died at 7.8 months from respiratory failure, which was deemed unrelated to treatment. One patient withdrew consent at 11.9 months of age; this patient was determined by the investigator to have required permanent ventilation at the time of discontinuation.2
dOne patient discontinued participation at the age of 18.0 months, before the month 18 end-of-study-visit, due to an adverse event of respiratory distress (considered unrelated to study drug). The patient was alive without permanent ventilation at the time of withdrawal.2
eNatural history: The Pediatric Neuromuscular Clinical Research (PNCR) Network study population, with bi-allelic deletion of SMN1 gene, 2 copies of SMN2, and onset of SMA symptoms at age ≤6 months, was used as a matched control cohort for START and STR1VE studies.3
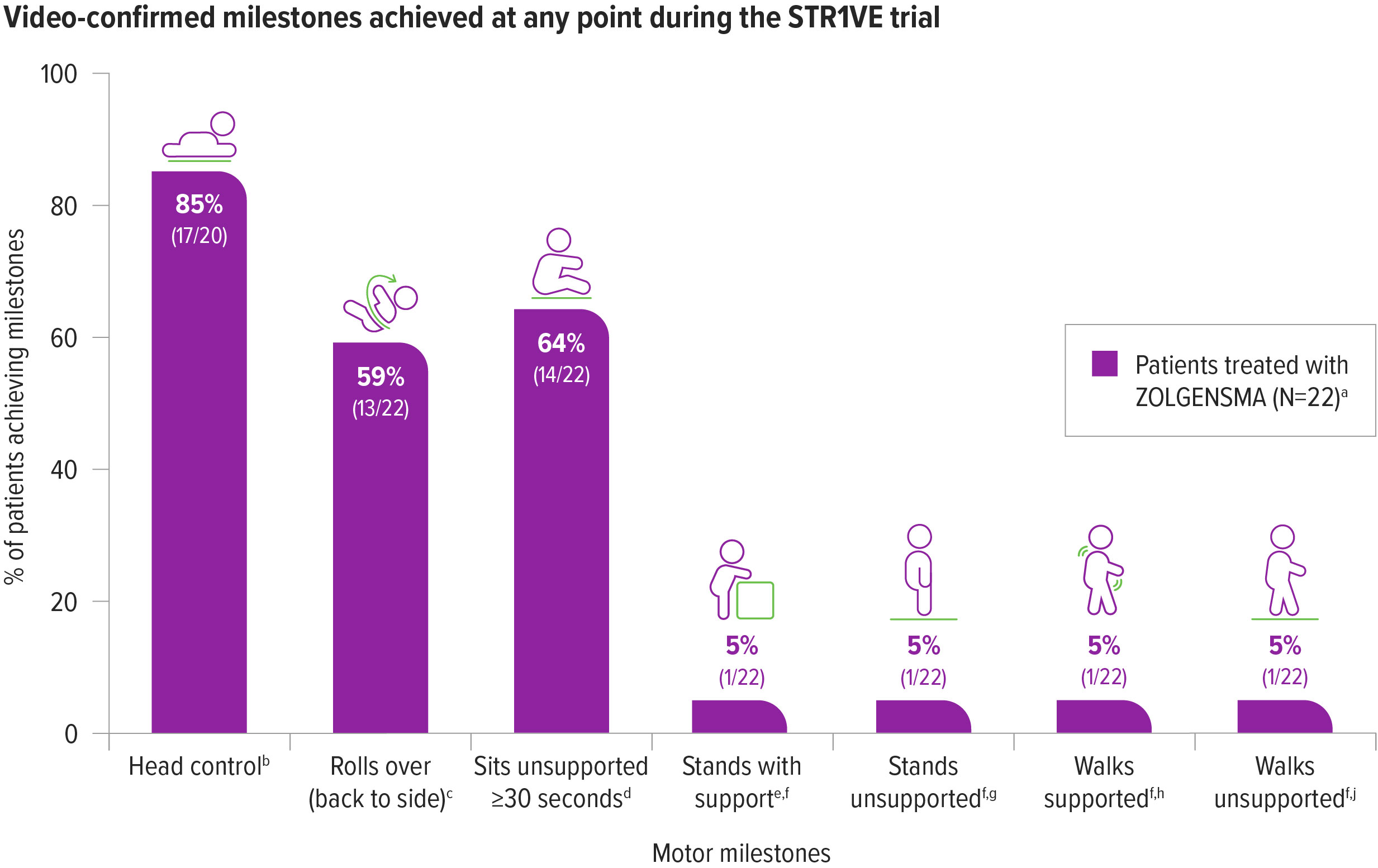
New motor milestones were achieved with ZOLGENSMA2
59% (13/22) of patients achieved sitting without support for ≥30 seconds at the 18-months-of-age study visit, a primary endpoint2
One additional patient achieved the milestone of sitting without support for ≥30 seconds at 16 months of age; however, this milestone was not reconfirmed at the 18-months-of-age visit.2
aOne patient was initially classified as presymptomatic and removed from the ITT data set included in the Prescribing Information. The patient has been confirmed to be symptomatic at the time of gene therapy and included in the final ITT analysis.2
bBayley-III, gross motor subtest item 4. Two patients demonstrated the milestone of head control at screening visit prior to ZOLGENSMA dosing; therefore n=20 was used for this calculation.2
cBayley-III, gross motor subtest item 20.2
dBayley-III, gross motor subtest item 26.2
eBayley-III, gross motor subtest item 33.2
fThese milestones were achieved by the same patient.2
gBayley-III, gross motor item 40.2
hBayley-III, gross motor item 37.2
iBayley-III, gross motor item 43.2
aOne patient was initially classified as presymptomatic and removed from the ITT data set included in the Prescribing Information. The patient has been confirmed to be symptomatic at the time of gene therapy and included in the final ITT analysis.2
bBayley-III, gross motor subtest item 4. Two patients demonstrated the milestone of head control at screening visit prior to ZOLGENSMA dosing; therefore n=20 was used for this calculation.2
cBayley-III, gross motor subtest item 20.2
dBayley-III, gross motor subtest item 26.2
eBayley-III, gross motor subtest item 33.2
fThese milestones were achieved by the same patient.2
gBayley-III, gross motor item 40.2
hBayley-III, gross motor item 37.2
iBayley-III, gross motor item 43.2
64% (14/22)of patients achieved sitting without support for ≥30 seconds, at any point during the STR1VE trial2,a,b
vs
In natural history, patients with SMA Type 1 are not able to sit unassisted4
aBayley-III, gross motor subtest item 26.2
bOne patient was initially classified as presymptomatic and removed from the intent-to-treat (ITT) data set included in the Prescribing Information. The patient has been later confirmed to be symptomatic at baseline and included in the final ITT analysis.2
ZOLGENSMA helped patients with SMA Type 1 maintain the ability to thrive2
41% (9/22) of patients met all 3 criteria for ability to thrive at 18 months of age, a secondary endpoint2,a,b
In natural history, most patients with SMA Type 1 older than 12 months of age required feeding support.3
aOne patient was initially classified as presymptomatic and removed from the intent-to-treat (ITT) data set included in the Prescribing Information. The patient has been confirmed to be symptomatic at the time of gene therapy and included in the final ITT analysis.
bAbility to thrive, a secondary endpoint, is defined as ability to tolerate thin liquids as demonstrated through a formal swallowing test, maintenance of weight (≥3rd percentile for age and gender as defined by WHO guidelines), and independence from mechanical or non-oral nutritional support.
Ability to thrive at 18 months of age was a composite endpoint consisting of2,a:
Swallowing thin liquids
55% (12/22) of patients
could swallow and tolerate thin liquidsa
Non-oral feeding support
86% (19/22) of patients
were free of ongoing non-oral feeding supporta
Maintenance of weight
64% (14/22) of patients
maintained weight consistent with age, at or above the 3rd percentilea
aOne patient was initially classified as presymptomatic and removed from the intent-to-treat (ITT) data set included in the Prescribing Information. The patient has been confirmed to be symptomatic at the time of gene therapy and is included in the final ITT analysis.
bAbility to thrive, a secondary endpoint, is defined as ability to tolerate thin liquids as demonstrated through a formal swallowing test, maintenance of weight (≥3rd percentile for age and gender as defined by WHO guidelines), and independence from mechanical or non-oral nutritional support.
ZOLGENSMA helped maintain respiratory status compared with natural history2,3
4/22 patients required ventilatory support as assessed by Trilogy BiPAP data alone at 18 months of age, a secondary endpoint2,a
In natural history, most patients with SMA Type 1 required respiratory support by 12 months of age.3
aOne patient was initially classified as presymptomatic and removed from the intent-to-treat (ITT) data set included in the Prescribing Information. The patient has been confirmed to be symptomatic at the time of gene therapy and included in the final ITT analysis.2
bFive patients had documented Trilogy BiPAP use, and two patients had other noninvasive ventilatory support.2
Noninvasive ventilatory support
68% (15/22) of patients
did not require noninvasive ventilatory support at any point during the study2,a,b
aOne patient was initially classified as presymptomatic and removed from the intent-to-treat(ITT) data set included in the Prescribing Information. The patient has been confirmed to be symptomatic at the time of gene therapy and is included in the final ITT analysis.2
bFive patients had documented Trilogy BiPAP use, and two patients had other noninvasive ventilatory support.2
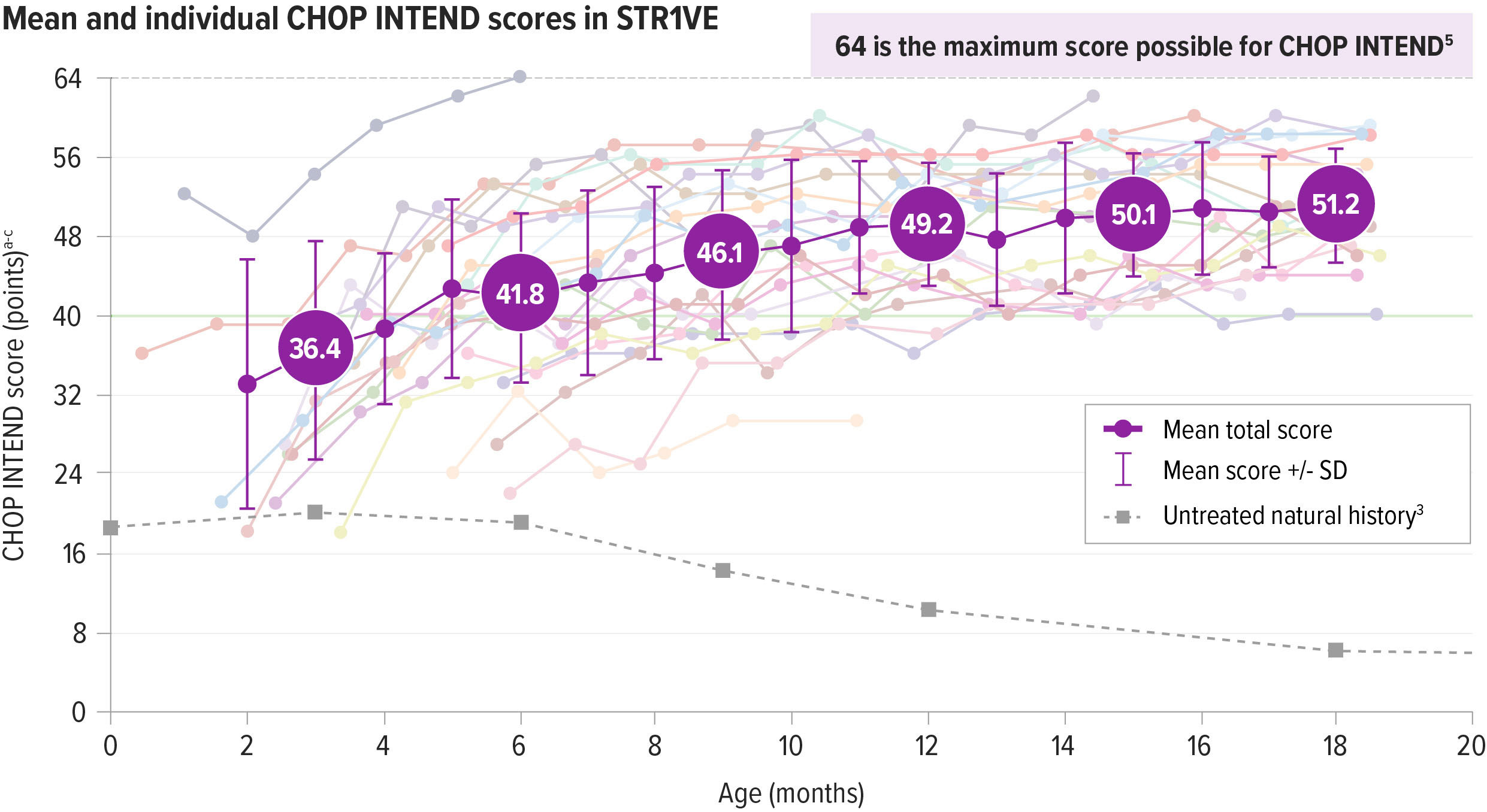
ZOLGENSMA resulted in maintained motor function improvements from baseline2
Motor function was assessed using the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND)
CHOP INTEND was specifically developed to assess motor function in patients with SMA. The CHOP INTEND score is measured in points, which are assigned based on a patient’s ability to perform specific motor skills. The test consists of 16 measures, each scored on a scale of 0-4, with the highest possible score being 64 points.5
Patients achieved CHOP INTEND scores not seen in natural history.2,6
Patients with SMA Type 1 in historical controls did not typically achieve and maintain CHOP INTEND scores of ≥40.6
At the end of STR1VE, nearly all patients (95%; 21/22) achieved or maintained a CHOP INTEND score of ≥402,a,b
SD=standard deviation.
Individual patient data are shown within the CHOP INTEND chart. Per protocol, patients who achieved 3 consecutive CHOP INTEND scores ≥58 did not undergo any additional CHOP INTEND examinations.
aOne patient was initially classified as presymptomatic and removed from the intent-to-treat (ITT) data set included in the Prescribing Information. The patient has been confirmed to be symptomatic at the time of gene therapy and included in the final ITT analysis.2
bScores on the CHOP INTEND scale of motor function range from 0 to 64, with higher scores indicating better function.5
cThe dark gray line at 40 represents the highest CHOP INTEND score that children with the most severe form of SMA would achieve or maintain, according to natural history.6
SD=standard deviation.
Individual patient data are shown within the CHOP INTEND chart. Per protocol, patients who achieved 3 consecutive CHOP INTEND scores ≥58 did not undergo any additional CHOP INTEND examinations.
aOne patient was initially classified as presymptomatic and removed from the intent-to-treat (ITT) data set included in the Prescribing Information. The patient has been confirmed to be symptomatic at the time of gene therapy and included in the final ITT analysis.2
bScores on the CHOP INTEND scale of motor function range from 0 to 64, with higher scores indicating better function.5
cThe dark gray line at 40 represents the highest CHOP INTEND score that children with the most severe form of SMA would achieve or maintain, according to natural history.6
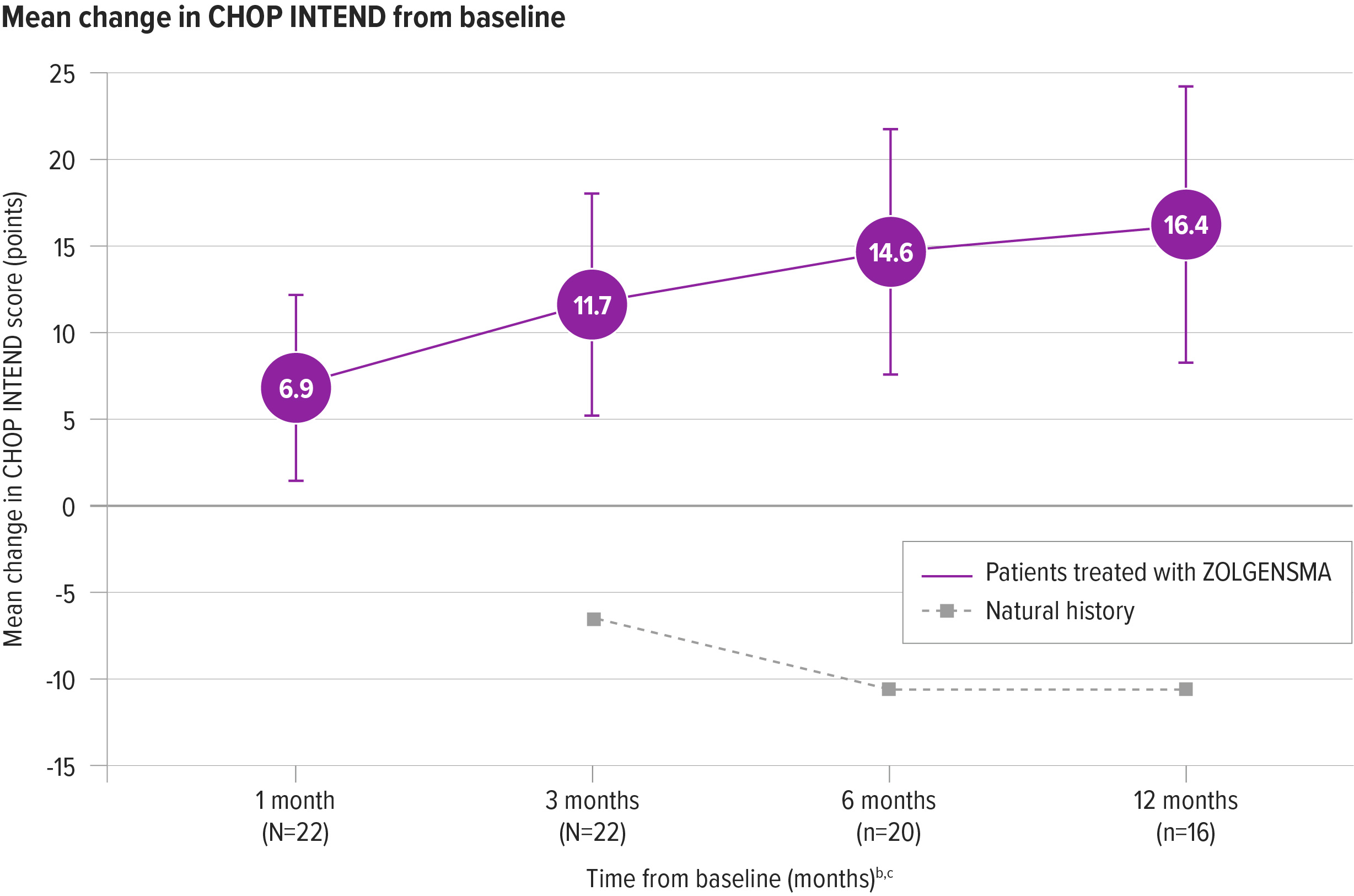
Rapid improvements in CHOP INTEND from baseline were seen as early as 1 month post infusion
One month after treatment, patients’ scores improved by a mean change of 6.9 points (N=22) from baseline. Improvements continued at 12 months, with a mean change of 16.4 points (n=16) from baseline2,a
aOne patient was initially classified as presymptomatic and removed from the ITT data set included in the Prescribing Information. The patient has been confirmed to be symptomatic at the time of gene therapy and included in the final ITT analysis.2
bBaseline for natural history was measured at 6 months of age (Kolb et al). Additional CHOP INTEND assessments were performed at 9, 12 and 18 months of age (3, 6 and 12 months from baseline). Baseline scores for ZOLGENSMA patients were performed prior to infusion and patients were assessed every month following infusion, through the duration of the study.7
cPer protocol, patients who achieved 3 consecutive CHOP INTEND scores ≥58 did not undergo any additional CHOP INTEND examinations.
aOne patient was initially classified as presymptomatic and removed from the ITT data set included in the Prescribing Information. The patient has been confirmed to be symptomatic at the time of gene therapy and included in the final ITT analysis.2
bBaseline for natural history was measured at 6 months of age (Kolb et al). Additional CHOP INTEND assessments were performed at 9, 12 and 18 months of age (3, 6 and 12 months from baseline). Baseline scores for ZOLGENSMA patients were performed prior to infusion and patients were assessed every month following infusion, through the duration of the study.7
cPer protocol, patients who achieved 3 consecutive CHOP INTEND scores ≥58 did not undergo any additional CHOP INTEND examinations.
START Trial
An open-label, single-arm, Phase 1, dose-escalation clinical trial supporting the efficacy of ZOLGENSMA
START TrialReferences: 1. Novartis Gene Therapies, Inc. Gene replacement therapy clinical trial for patients with spinal muscular atrophy type 1 (STR1VE). https://clinicaltrials.gov/study/NCT03306277. ClinicalTrials.gov identifier: NCT03306277. Updated August 16, 2022. Accessed October 19, 2023. 2. Data on file. AveXis, Inc. 2020. 3. Finkel RS, McDermott MP, Kaufmann P, et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83(9):810-817. 4. De Sanctis R, Coratti G, Pasternak A, et al. Developmental milestones in type I spinal muscular atrophy. Neuromuscul Disord. 2016;26(11):754-759. 5. Novartis Gene Therapies, Inc. single-dose gene replacement therapy clinical trial for participants with spinal muscular atrophy Type 1 (STRIVE-EU). https://clinicaltrials.gov/study/NCT03461289. ClinicalTrials.gov identifier: NCT03461289. Updated August 16, 2022. Accessed October 19, 2023. 6. Glanzman AM, Mazzone E, Main M, et al. The Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND): test development and reliability. Neuromuscul Disord. 2010;20(3):155-161. 7. Lowes LP, Alfano LN, Arnold WD, et al. Impact of age and motor function in a phase 1/2A study of infants with SMA type 1 receiving single-dose gene replacement therapy. Pediatr Neurol. 2019;98:39-45. 8. Kolb SJ, Coffey CS, Yankey JW, et al; NeuroNEXT Clinical Trial Network on behalf of the NN101 SMA Biomarker Investigators. Natural history of infantile-onset spinal muscular atrophy. Ann Neurol. 2017;82(6):883-891.