Efficacy: START trial
START: An open-label, single-arm, dose-escalation clinical trial supports the efficacy of ZOLGENSMA1,2
START was a Phase 1, 2-year (24-month) study of ZOLGENSMA that enrolled 15 patients with SMA Type 1
Symptomatic patients with onset of clinical symptoms before 6 months of age were divided into 2 cohorts—a low-dose cohort (n=3) and a high-dose cohort (n=12).1,a
aThe dosage received by patients in the low-dose cohort was approximately one-third of the dosage received by patients in the high-dose cohort. However, the precise dosages of ZOLGENSMA received by patients in this completed clinical trial are unclear due to a change in the method of measuring ZOLGENSMA concentration, and to decreases in the concentration of stored ZOLGENSMA over time.2
EXPAND ENDPOINTS AND ENROLLMENT CRITERIA
Endpoints1
Primary endpoint:
- 24-month safety
Secondary endpoints:
-
Event-free survivala
- Change from baseline in Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND) scores
Other endpoints:
- Motor milestone and developmental achievements
aEvent is defined as death or the need for permanent ventilatory support consisting of ≥16 hours of respiratory assistance per day continuously for ≥14 days in the absence of an acute reversible illness, excluding perioperative ventilation.
Key inclusion and exclusion criteria1
Key inclusion criteria:
- Patients were ≤9 months of age (first 9 patients) or ≤6 months of age (last 6 patients)
- Bi-allelic SMN1 mutations (deletion or point mutations) and 2 copies of SMN2
Key exclusion criteria:
-
Genetic modifier c.859G>C in SMN2 exon 7
- Anti-AAV9 antibody titers >1:50
- Active viral infection
- Use of invasive ventilatory support or pulse oximetry <95% saturation
- Signs of aspiration based on a swallowing test and unwilling to use an alternative method to oral feeding
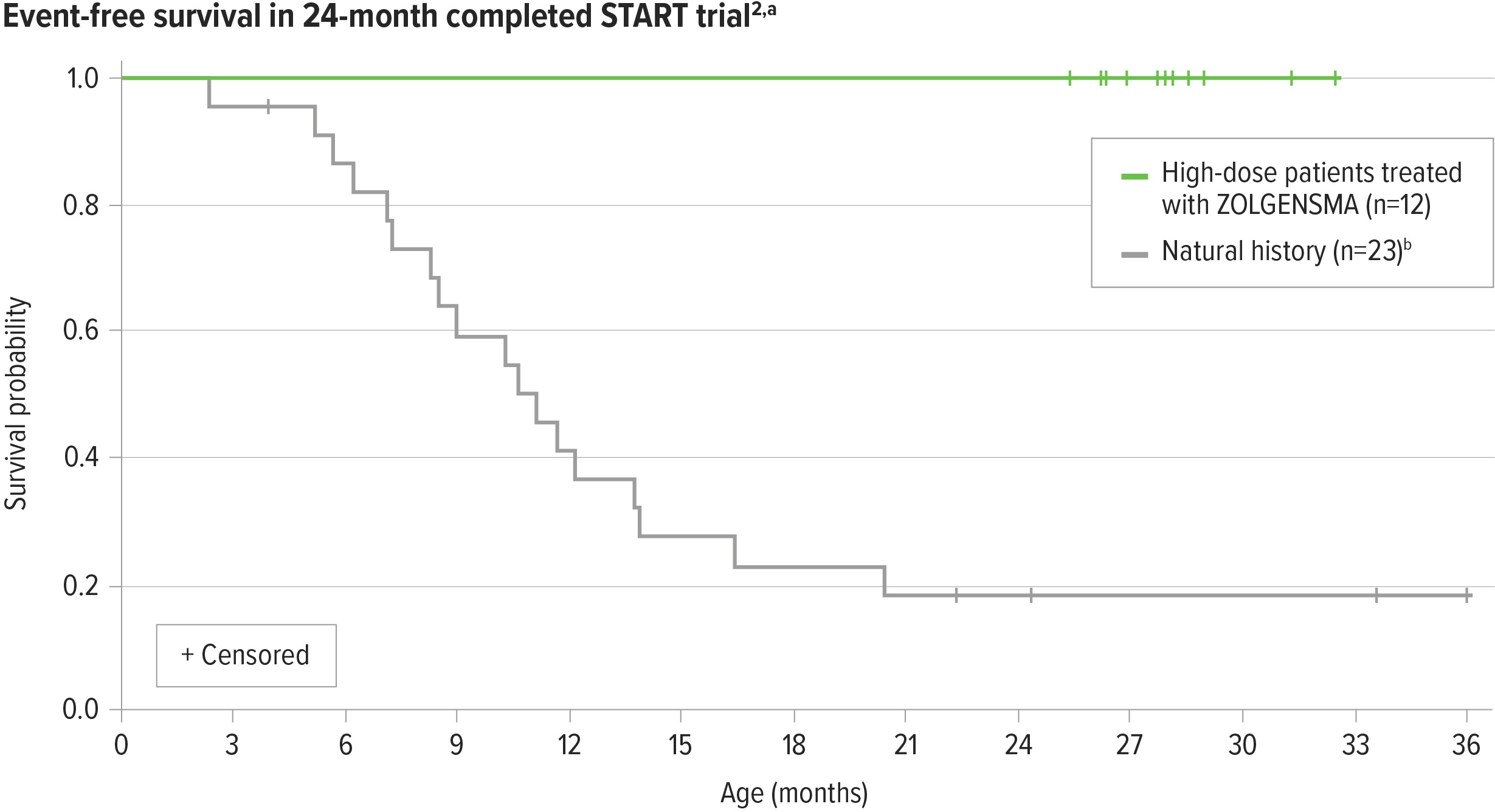
ZOLGENSMA significantly increased event-free survival in patients from the high-dose cohort compared with natural history2-4,a-c
100% (12/12) of patients in the high-dose cohort were alive and free of permanent ventilation at 24 months post infusion3
aEvent is defined as death or the need for permanent ventilatory support consisting of ≥16 hours of respiratory assistance per day continuously for ≥14 days.2
bNatural history: The Pediatric Neuromuscular Clinical Research (PNCR) Network study population, with bi-allelic deletion of SMN1 gene, 2 copies of SMN2, and onset of SMA symptoms at age ≤6 months, was used as a matched control cohort for START and STR1VE studies.4
cHigh-dose cohort (n=12) in a 24-month clinical trial of ZOLGENSMA.
aEvent is defined as death or the need for permanent ventilatory support consisting of ≥16 hours of respiratory assistance per day continuously for ≥14 days.2
bNatural history: The Pediatric Neuromuscular Clinical Research (PNCR) Network study population, with bi-allelic deletion of SMN1 gene, 2 copies of SMN2, and onset of SMA symptoms at age ≤6 months, was used as a matched control cohort for START and STR1VE studies.4
cHigh-dose cohort (n=12) in a 24-month clinical trial of ZOLGENSMA.
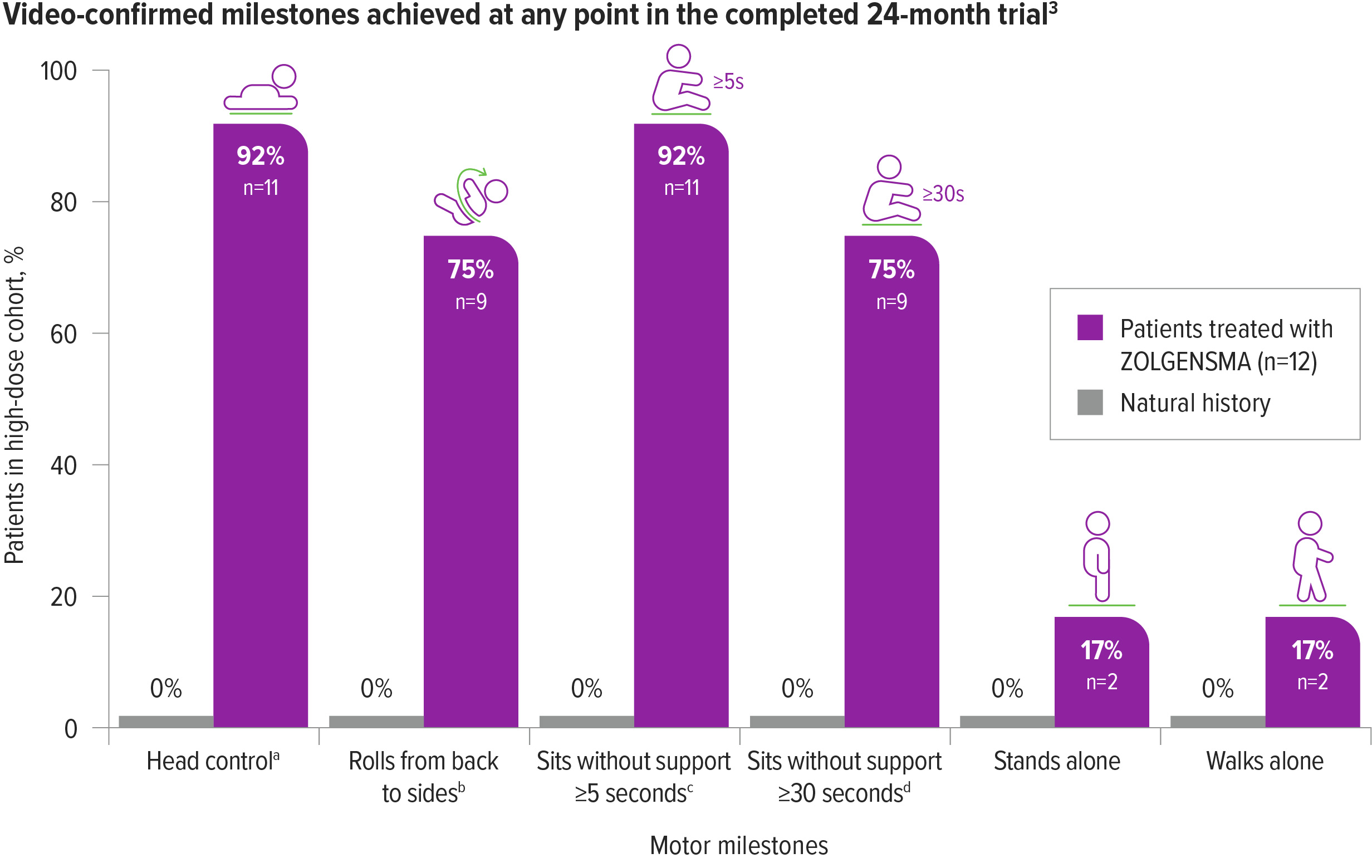
Motor milestones were achieved and maintained with ZOLGENSMA in patients from the high-dose cohort3,a-d
75% (9/12) of patients could sit without support for ≥30 seconds by the end of the 24-month study3,c,d
In natural history, 0% of patients with SMA Type 1 were able to sit unassisted.5
aBayley-III, gross motor subtest item 20.3
bBayley-III, gross motor subtest item 22.3
cBayley-III, gross motor subtest item 26.3
dAlso includes patients who are observed sitting alone for ≥5, ≥10, or ≥30 seconds. Patients sitting without support ≥30 seconds are included in the total of ≥5 seconds.3
aBayley-III, gross motor subtest item 20.3
bBayley-III, gross motor subtest item 22.3
cBayley-III, gross motor subtest item 26.3
dAlso includes patients who are observed sitting alone for ≥5, ≥10, or ≥30 seconds. Patients sitting without support ≥30 seconds are included in the total of ≥5 seconds.3
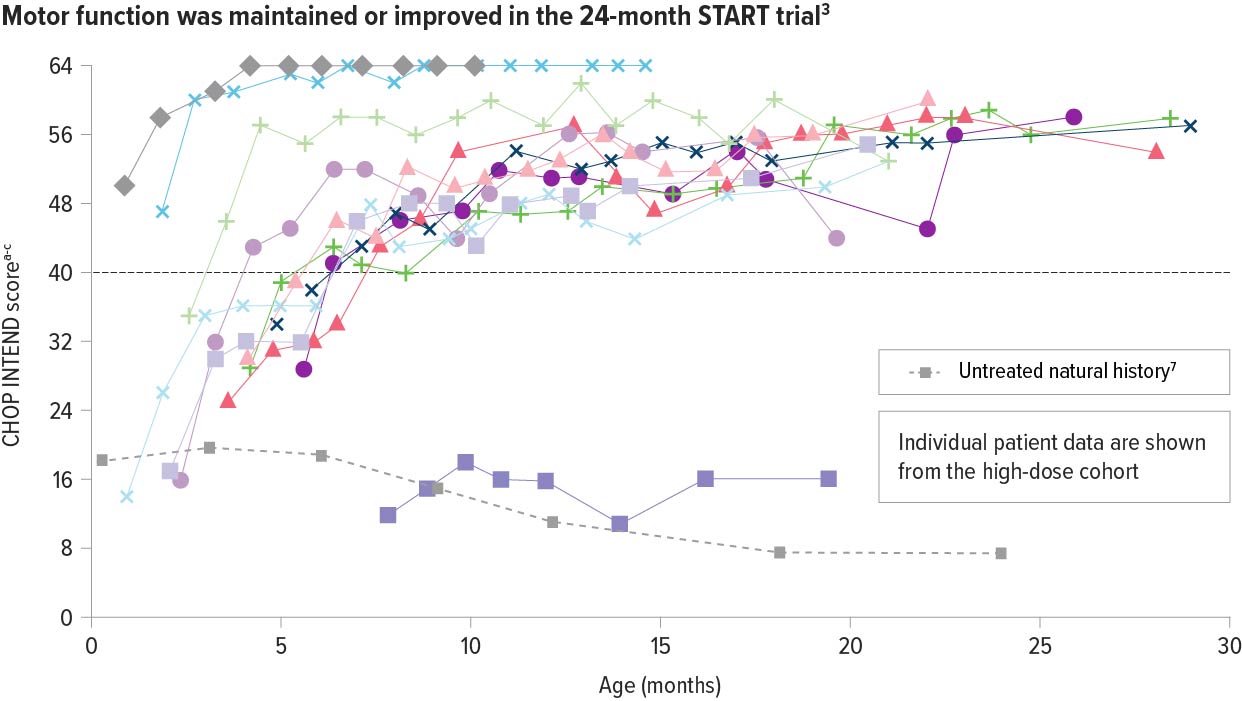
In the high-dose cohort, ZOLGENSMA significantly improved motor function as early as 1 month post infusion3
Patients with SMA Type 1 in historical controls do not typically achieve CHOP INTEND scores ≥40.6
92% (11/12) of patients achieved or maintained a CHOP INTEND score of ≥40 points6,a,b
Motor function was assessed using the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND)
CHOP INTEND was specifically developed to assess motor function in patients with SMA. The CHOP INTEND score is measured in points, which are assigned based on a patient’s ability to perform specific motor skills. The test consists of 16 measures, each scored on a scale of 0-4, with the highest possible score being 64 points.8
aHigh-dose cohort (n=12) in START: a completed, 24-month, open-label, single-arm, ascending-dose clinical trial of ZOLGENSMA.
bScores on the CHOP INTEND scale of motor function range from 0 to 64, with higher scores indicating better function.8
cPatients who achieved 2 consecutive scores 62 or higher did not undergo additional CHOP INTEND tests.
aHigh-dose cohort (n=12) in START: a completed, 24-month, open-label, single-arm, ascending-dose clinical trial of ZOLGENSMA.
bScores on the CHOP INTEND scale of motor function range from 0 to 64, with higher scores indicating better function.8
cPatients who achieved 2 consecutive scores 62 or higher did not undergo additional CHOP INTEND tests.
ZOLGENSMA helped maintain nutritional and respiratory status over 24 months compared with natural history3,4
In the natural history of SMA Type 1, nearly all patients required nutritional support and most patients required respiratory support by 12 months of age.4
aHigh-dose cohort (n=12) in START: a completed, 24-month, open-label, single-arm, ascending-dose clinical trial of ZOLGENSMA.
bOne additional patient with no BiPAP prior to gene therapy continues without regular BiPAP, but uses when ill.3
Results at the end of the 24-month study
Respiratory status
60% (6/10) of patients
with no BiPAP support prior to gene therapy continued without BiPAP support3,a,b
Nutritional status
86% (6/7) of patients
who did not require feeding support before gene therapy continued without nutritional support3,a
aHigh-dose cohort (n=12) in START: a completed, 24-month, open-label, single-arm, ascending-dose clinical trial of ZOLGENSMA.
bOne additional patient with no BiPAP prior to gene therapy continues without regular BiPAP, but uses when ill.3
ZOLGENSMA helped sustain bulbar function in patients from the high-dose cohort
aHigh-dose cohort (n=12) in START: a completed, 24-month, open-label, single-arm, dose-escalation clinical trial of ZOLGENSMA.
bSwallow function was last assessed at 24 months, however, one patient had the last assessment at 12 months.3
Bulbar function
92% (11/12) of patients
could speak, swallow, and feed orally at last assessment3,a,b
aHigh-dose cohort (n=12) in START: a completed, 24-month, open-label, single-arm, dose-escalation clinical trial of ZOLGENSMA.
bSwallow function was last assessed at 24 months, however, one patient had the last assessment at 12 months.3
References: 1. Novartis Gene Therapies, Inc. Gene transfer clinical trial for spinal muscular atrophy type 1. https://clinicaltrials.gov/study/NCT02122952. ClinicalTrials.gov identifier: NCT02122952. Updated September 15, 2022. Accessed October 19, 2023. 2. ZOLGENSMA [prescribing information]. Bannockburn, IL: Novartis Gene Therapies, Inc; 2023. 3. Data on file. AveXis, Inc. 2019. 4. Finkel RS, McDermott MP, Kaufmann P, et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83(9):810-817. 5. De Sanctis R, Coratti G, Pasternak A, et al. Developmental milestones in type I spinal muscular atrophy. Neuromuscul Disord. 2016;26(11):754-759. 6. Lowes LP, Alfano LN, Arnold WD, et al. Impact of age and motor function in a phase 1/2A study of infants with SMA type 1 receiving single-dose gene replacement therapy. Pediatr Neurol. 2019;98:39-45. 7. Kolb SJ, Coffey CS, Yankey JW, et al; NeuroNEXT Clinical Trial Network on behalf of the NN101 SMA Biomarker Investigators. Natural history of infantile-onset spinal muscular atrophy. Ann Neurol. 2017;82(6):883-891. 8. Glanzman AM, Mazzone E, Main M, et al. The Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND): test development and reliability. Neuromuscul Disord. 2010;20(3):155-161.