ZOLGENSMA is a gene replacement therapy designed to treat the genetic root cause of SMA1
Spinal muscular atrophy (SMA) is caused by the deletion or mutation of the survival motor neuron 1 (SMN1) gene. The SMN1 gene produces survival motor neuron (SMN) protein that is critical for function of motor neurons. Patients with SMA have an insufficient amount of SMN protein, which leads to permanent loss of motor neurons.2,3
ZOLGENSMA is designed to enable rapid and continuous expression of SMN protein. By replacing the missing SMN protein, ZOLGENSMA can stop the progressive loss of motor neurons.1,4
The key elements of ZOLGENSMA
Key components of ZOLGENSMA enable widespread SMN protein expression in the central nervous system and in motor neurons.
AAV9 vector
Crosses the blood-brain barrier and enters motor neurons1,5
Continuous promoter
Enables ongoing expression of the SMN gene1,6
SMN transgene
Contains the genetic code for the SMN protein patients need1
How ZOLGENSMA works
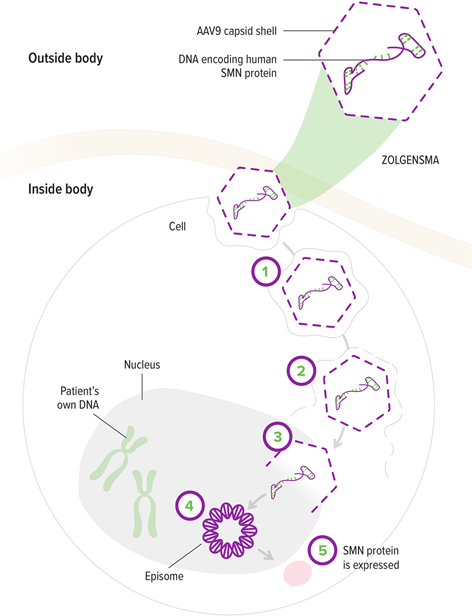
1. The AAV9 vector enters motor neurons1
2. The AAV9 vector delivers the SMN gene to the cell nucleus1,5
3. The SMN gene is introduced to target cells as recombinant, self-complementary DNA1
4. The self-complementary ends form a circular episome that can persist in the nucleus of motor neuron cells. These cells are nondividing5,6
5. This results in rapid activation and continuous expression of the SMN gene, leading to the production of SMN protein4
See how ZOLGENSMA enters motor neurons
VO
ZOLGENSMA (onasemnogene abeparvovec-xioi) is the first one-time-only gene therapy for the treatment of pediatric patients less than 2 years of age with SMA.
ZOLGENSMA has a Boxed Warning for Serious Liver Injury and Acute Liver Failure. Please see additional Important Safety Information at the end of this video. Please also see the Full Prescribing Information at ZOLGENSMA-hcp.com.
VO
SMA is a progressive and neuromuscular disease resulting from the bi-allelic deletion or mutation in the survival motor neuron 1, or SMN1, gene.
SMN1 is the only gene that consistently produces SMN protein, which is critical for neuronal survival.
SMN1 has a backup gene, SMN2, whose splicing variability results in approximately 10% functional SMN protein.
Without functional SMN1, patients with SMA rely solely on the insufficient levels of protein produced by SMN2.
SMN2 copy number is an important modifier of disease severity in SMA.
In patients with SMA, there is significant variability in SMN2 copy number. Those with the most severe forms of SMA often have 1-2 copies of SMN2.
Without sufficient SMN protein production, patients experience irreversible neuronal loss, resulting in progressive muscle atrophy, which may lead to physical disability, life-threatening medical emergencies, and may result in premature death.
ZOLGENSMA is a gene therapy that stops SMA progression with a one-time-only dose. It targets the genetic root cause of SMA, mutations in the SMN1 gene, by introducing a functional copy of the human SMN gene.
To combat the neurodegenerative nature of the disease, ZOLGENSMA is designed for rapid and continuous SMN protein production, which can stop the progression of disease and preserve motor neurons.
ZOLGENSMA is comprised of a functioning copy of the human SMN gene, which codes for the SMN protein patients need.
ZOLGENSMA has self-complementary ends that form double-stranded loops and has a promoter that activates the human SMN gene and initiates rapid and continuous protein expression.
The genetic elements of ZOLGENSMA are packaged in an adeno-associated virus, serotype 9 or AAV9, vector, which enables delivery of the gene into motor neurons and other, non-CNS tissues.
The AAV9 vector is nonpathogenic, and the viral genes have been removed to decrease immunogenic potential and eliminate potential viral replication. AAV9 vectors are not known to cause disease in humans.
ZOLGENSMA is administered as an intravenous infusion.
Once in the body, the AAV9 vector is able to cross the blood-brain barrier.
Having crossed the blood-brain barrier, the AAV9 vector can efficiently enter motor neuron cells.
Once inside the cell nucleus, the AAV9 vector releases the human SMN gene. The ZOLGENSMA SMN gene is designed to replace the function of the defective SMN1 gene.
The SMN gene is introduced to target cells as recombinant, self-complementary DNA.
The self-complementary ends of ZOLGENSMA are designed to form a circular episome that can persist in the nucleus of motor neuron cells, which are nondividing.
The self-complementary DNA and continuous promoter allow for ZOLGENSMA to be a one-time treatment by enabling rapid activation and continuous expression of the SMN transgene.
By targeting motor neurons throughout the CNS ZOLGENSMA stops the widespread neuronal cell death and subsequent muscle degeneration characteristic of SMA, preserving motor neurons and sustaining neuromuscular function.
VOICE-OVER
Indication and Important Safety Information for ZOLGENSMA (onasemnogene abeparvovec-xioi)
INDICATION
ZOLGENSMA is an adeno-associated virus (AAV) vector-based gene therapy indicated for the treatment of pediatric patients less than 2 years of age with spinal muscular atrophy (SMA) with bi-allelic mutations in the survival motor neuron 1 (SMN1) gene.
Limitations of Use
The safety and effectiveness of repeat administration or the use in patients with advanced SMA (eg, complete paralysis of limbs, permanent ventilator dependence) has not been evaluated with ZOLGENSMA.
IMPORTANT SAFETY INFORMATION
BOXED WARNING: Serious Liver Injury and Acute Liver Failure
Cases of acute liver failure with fatal outcomes have been reported. Acute serious liver injury, acute liver failure, and elevated aminotransferases can also occur with ZOLGENSMA. Patients with preexisting liver impairment may be at higher risk. Prior to infusion, assess liver function of all patients by clinical examination and laboratory testing. Administer systemic corticosteroid to all patients before and after ZOLGENSMA infusion. Continue to monitor liver function for at least 3 months after infusion, and at other times as clinically indicated. If acute serious liver injury or acute liver failure is suspected, promptly consult a pediatric gastroenterologist or hepatologist.
WARNINGS AND PRECAUTIONS
Systemic Immune Response Patients with underlying active infection, either acute or chronic uncontrolled, could be at an increased risk of serious systemic immune response. Administer ZOLGENSMA to patients who are clinically stable in their overall health status (eg, hydration and nutritional status, absence of infection). Postpone ZOLGENSMA in patients with infections until the infection has resolved and the patient is clinically stable.
Thrombocytopenia
Transient decreases in platelet counts, some of which met the criteria for thrombocytopenia, were typically observed within the first 2 weeks after ZOLGENSMA infusion. Monitor platelet counts before ZOLGENSMA infusion and on a regular basis for at least 3 months afterwards.
Thrombotic Microangiopathy
Cases of thrombotic microangiopathy (TMA) were reported to occur generally within the first 2 weeks after ZOLGENSMA infusion. TMA can result in life-threatening or fatal outcomes. Obtain baseline creatinine and complete blood count before ZOLGENSMA infusion. Following infusion, monitor platelet counts closely as well as other signs and symptoms of TMA. Consult a pediatric hematologist and/or pediatric nephrologist immediately to manage as clinically indicated.
Elevated Troponin I
Increases in cardiac troponin I levels have occurred following ZOLGENSMA infusion. Consider cardiac evaluation after ZOLGENSMA infusion and consult a cardiologist as needed.
AAV Vector Integration and Risk of Tumorigenicity
There is a theoretical risk of tumorigenicity due to integration of AAV vector DNA into the genome. Cases of tumor have been reported in patients who received ZOLGENSMA post-approval; a causal relationship has not been established based on tumor analysis. In some cases, limited information was available. Report cases of tumor development in patients who received ZOLGENSMA to Novartis Gene Therapies, Inc. at 1-833-828-3947.
Infusion-Related Reactions
Infusion-related reactions, including hypersensitivity reactions and anaphylaxis, have occurred with ZOLGENSMA infusion. Signs and symptoms may include rash, urticaria, vomiting, dyspnea, respiratory symptoms, and/or alterations in heart rate and blood pressure. Monitor patients during and after treatment with ZOLGENSMA. If an infusion-related reaction occurs, interrupt ZOLGENSMA infusion and administer supportive treatment to manage the infusion-related reaction as appropriate. Infusion of ZOLGENSMA may be resumed based on clinical assessment.
ADVERSE REACTIONS
The most commonly observed adverse reactions (incidence ≥5%) in clinical studies were elevated aminotransferases and vomiting.
Please see Full Prescribing Information.
