Efficacy: LTFU
START Long-Term Follow-Up (LTFU): ZOLGENSMA demonstrates durability of effect up to 7.5 years after treatment1
An ongoing, long-term follow-up study of patients from the START clinical trial (N=13)1,2
The long-term follow-up study of START began after the completion of the 24-month START trial and is planned to last 15 years. A total of 13 patients, 10 from the high-dose cohort and 3 from the low-dose cohort, are enrolled in the study.
EXPAND ENDPOINTS AND ENROLLMENT CRITERIA
Endpoints2
Primary endpoint:
- Long-term safety as assessed by incidence of serious adverse events (SAEs) and adverse events (AEs) of special interest
Key inclusion and exclusion criteria2
Key inclusion criteria:
- Received ZOLGENSMA in the START trial for SMA Type 1
- Parent/legal guardian willing and able to complete the informed consent process, comply with study procedures, and visit schedule
Key exclusion criteria:
- Parent/legal guardian unable or unwilling to participate in the long-term follow-up safety study

The latest START long-term follow-up data
(May 2022 data cut)
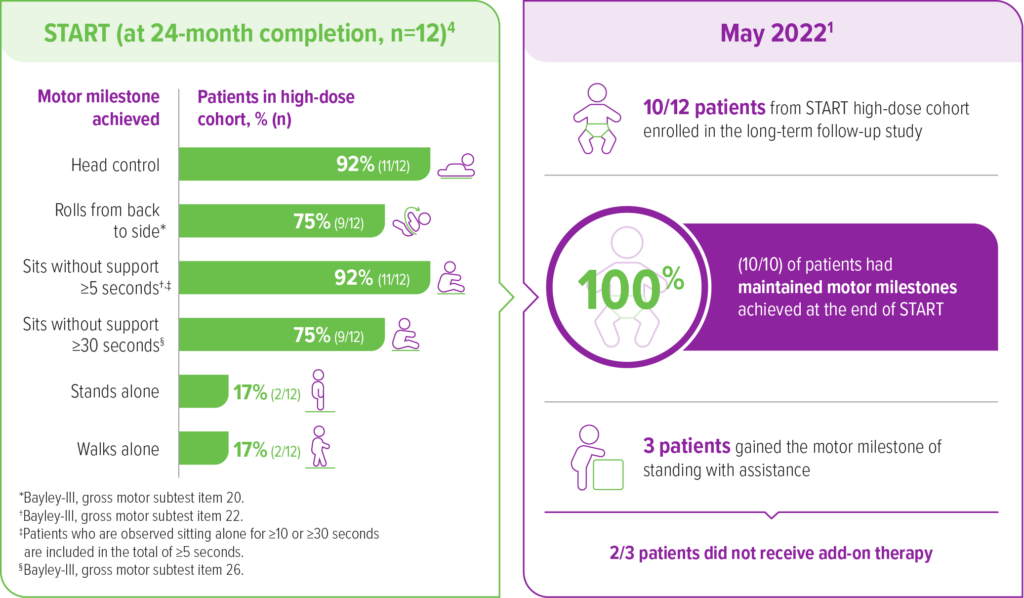
A total of 10 out of 12 high-dose patients enrolled in an ongoing, observational, long-term follow-up of the START trial1
As of May 2022, the mean time since treatment was 6.9 years (range 6.4-7.5) and the mean age at last follow up was 7.1 years (range 6.6-7.9).1,a
aA 15-year, voluntary follow-up of patients in the START trial who received a high dose of ZOLGENSMA (n=10). In long-term follow-up, 6 patients had used another disease-modifying therapy.1
As of the May 2022 data cut1:
Event-free survival
100% (10/10) of patients
were alive and free of permanent ventilation1,a
ZOLGENSMA continues to provide durable efficacy up to 7.5 years after treatment1
The long-term follow-up study is an ongoing follow-up of the Phase 1 START trial.
All 10 patients from the START high-dose cohort who enrolled in the long-term follow-up were alive and free of permanent ventilation as of May 20221
aAs of the last visit prior to December 2019 data cut.
bBased on adverse event reporting death, withdrawal, or permanent ventilation. There were no in-person assessments between December 2019 and June 2020.
START Trial
An open-label, single-arm, Phase 1, dose-escalation clinical trial supported the efficacy of ZOLGENSMA
START TrialReferences: 1. Data on file. Novartis Gene Therapies, Inc. 2023. 2. Novartis Gene Therapies, Inc. Long-term follow-up study for patients from AVXS-101-CL-101 (START). https://clinicaltrials.gov/study/NCT03421977. ClinicalTrials.gov identifier: NCT03421977. Updated July 11, 2023. Accessed October 19, 2023. 3. Finkel RS, McDermott MP, Kaufmann P, et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83(9):810-817. 4. Data on file. AveXis, Inc. 2020.
